Magnesium battery prototype operates at room temperature
To address the growing need for large-scale, sustainable energy storage, researchers at Tohoku University have developed a prototype rechargeable magnesium battery (RMB) that overcomes many of the challenges faced by magnesium-based energy storage. This development represents a potential next stage in energy storage — a fast-charging battery made from sustainable materials.
Lithium is a scarce resource, which makes it difficult to produce enough lithium-ion batteries to keep up with new technology. In comparison, magnesium can be found in abundance, in the Earth’s crust. Tetsu Ichitsubo, from Tohoku University, said the reason magnesium hasn’t been the main material used for batteries is because of a sluggish reaction that prevents room-temperature operation.
“Imagine if your device batteries could only function in extreme temperatures. It would be essentially useless for day-to-day life,” Ichitsubo said.
Achieving room-temperature operation is key to realising magnesium-based energy storage as a competitive alternative that can reduce dependence on limited lithium resources. Using a newly designed amorphous oxide cathode (Mg0.27Li0.09Ti0.11Mo0.22O), the researchers achieved this feat.
Previous magnesium batteries had issues achieving fast and reversible Mg-ion diffusion, which prevented them from operating efficiently at room temperature. However, the amorphous oxide cathode uses an ion-exchange process between lithium and magnesium that creates diffusion pathways that allow Mg ions to move easily. As a result, the cathode supports reversible magnesium insertion and extraction at room temperature.
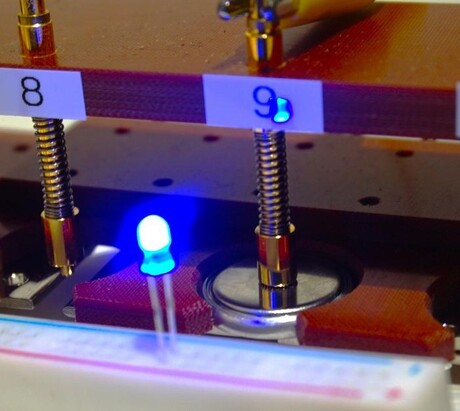
“We made a prototype full cell to test this battery in action, and found it was able to discharge sufficient amounts of energy even after 200 cycles,” Ichitsubo said, “It was enough to continuously power a blue light-emitting diode (LED). This is exciting, because previous demonstrations of RMBs showed negative discharge voltages, which means they failed to deliver usable energy.”
The researchers investigated the underlying mechanism of this battery. The study confirmed that the observed capacity originates from true magnesium intercalation, verified by rigorous chemical analysis. This distinguishes the system from previous reports where side reactions, rather than Mg-ion movement, dominated the apparent performance.
The study demonstrates an oxide cathode enabling RMB operation under ambient conditions and establishes fundamental design principles for next-generation cathode materials, introducing structural free volume, controlling particle size at the nanoscale and ensuring compatibility with advanced electrolytes.
These advances bring RMBs closer to practical application as safe, sustainable and resource-resilient energy storage systems. The research findings have been published in the journal Communications Materials.
Molecular Velcro coating boosts solar cell efficiency
Researchers have developed a new coating layer that enhances the durability and efficiency of...
Predictive AI model enhances solid-state battery design
ECU researchers are working on ways to make solid-state batteries more reliable with the help of...
Boosting performance of aqueous zinc–iodine batteries
Engineers from the University of Adelaide have enhanced aqueous zinc–iodine batteries using...




