New storage mechanism for cathodes in Na-ion batteries

Lithium-ion (Li-ion) and sodium-ion (Na-ion) batteries operate through a process called intercalation, where ions are stored and exchanged between two chemically different electrodes. In contrast, co-intercalation, a process in which both ions and solvent molecules are stored simultaneously, has traditionally been considered undesirable due to its tendency to cause rapid battery failure.
Against this traditional view, a research team led by Professor Philipp Adelhelm has now demonstrated that co-intercalation can be a reversible and fast process for cathode materials in Na-ion batteries. The approach of jointly storing ions and solvents in cathode materials provides a new handle for designing batteries with high efficiency and fast charging capabilities. The results have been published in the journal Nature Materials.
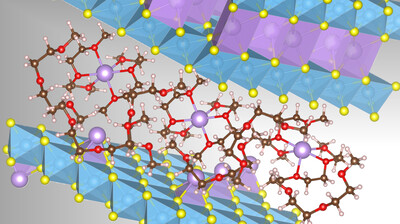
The performance of batteries depends on many factors. In particular, it depends on how ions are stored in electrode materials and whether they can be released again. This is because the charge carriers (ions) are relatively large and can cause an undesirable change in volume when they migrate into the electrode. This effect, known as ‘breathing’, impairs the battery’s service life. The volume change is particularly pronounced when sodium ions migrate together with molecules from the organic electrolyte. This so-called co-intercalation has generally been considered detrimental to battery life. However, the research team led by Adelhelm has now investigated cathode materials that enable the co-intercalation of ions and solvent molecules, allowing for faster charging and discharging processes.
In earlier studies, the team investigated co-intercalation in graphite anodes, demonstrating that sodium could migrate quickly and reversibly into and out of the electrolyte over many cycles when combined with glyme molecules. Nevertheless, proofing the same concept for cathode materials remained difficult. To tackle this challenge, the team explored a range of layered transition metal sulfides and identified solvent co-intercalation processes in cathode materials. “The process of co-intercalation could be used for developing very efficient and faster-charging batteries. This is why we wanted to investigate this topic in more detail,” Adelhelm said.
The study incorporates investigations from the last three years: Dr Yanan Sun carried out volume change measurements in the cathode materials, performed structural analyses with synchrotron radiation at PETRA III at DESY, and investigated the electrochemical properties for a variety of combinations of electrodes and solvents. By support from theory, in collaboration with Dr Gustav Åvall, important parameters could be identified that help in predicting co-intercalation reactions in the future.
“The co-intercalation process in cathode materials differs significantly from what happens in graphite anodes,” Sun said. While co-intercalation reactions in graphite anodes typically result in low-capacity electrodes, the loss of capacity caused by co-intercalation in the investigated cathode materials is very low. “Above all, certain cathode materials offer a huge advantage: the kinetics are super-fast, almost like a supercapacitor!” Sun said.
“The true beauty of co-intercalation reactions lies in their ability to offer a vast chemical landscape for designing novel layered materials for diverse applications. Exploring the concept of co-intercalation was extremely risky because it is against classical battery knowledge. I was therefore very grateful to receive funding for this idea from the European Research Council through an ERC Consolidator Grant,” Adelhelm said.
Molecular Velcro coating boosts solar cell efficiency
Researchers have developed a new coating layer that enhances the durability and efficiency of...
Predictive AI model enhances solid-state battery design
ECU researchers are working on ways to make solid-state batteries more reliable with the help of...
Boosting performance of aqueous zinc–iodine batteries
Engineers from the University of Adelaide have enhanced aqueous zinc–iodine batteries using...