Solid sodium battery has improved storage and safety
Seeking to satisfy the growing demand for light, powerful and long-lasting batteries, Swiss researchers have developed an initial prototype of a solid sodium battery with the potential to store extra energy. Described in the journal Energy & Environmental Science, their battery is based on sodium — a cheaper and arguably safer alternative to lithium.
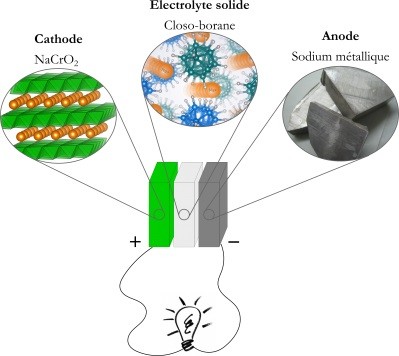
In order for a battery to work, it must have the following three key components: an anode (the negative pole), a cathode (the positive pole) and an electrolyte. Most of the batteries used in electronic equipment today are based on lithium ions. When the battery charges, the lithium ions leave the cathode and move to the anode. To prevent lithium dendrites forming — a kind of microscopic stalagmite that can induce short circuits in the battery that may cause fire — the anode in commercial batteries is made from graphite rather than metallic lithium, even though this ultralight metal would increase the amount of energy that can be stored.
Researchers from the University of Geneva (UNIGE) and Empa, the Swiss Federal Laboratories for Materials Science and Technology, set out to create a new prototype focused on the advantages of a ‘solid’ battery to cope with the heightened demand and to increase performance, aiming for faster charging together with increased storage capacity and improved safety. Their battery uses a solid instead of a liquid electrolyte that enables the use of a metal anode by blocking the formation of dendrites, making it possible to store more energy while ensuring safety.
“But we still had to find a suitable solid ionic conductor that, as well as being non-toxic, was chemically and thermally stable, and that would allow the sodium to move easily between the anode and the cathode,” noted Professor Hans Hagemann, a co-author on the study from UNIGE’s Faculty of Sciences.
The researchers discovered that a boron-based substance, a closo-borane, enabled the sodium ions to circulate freely. Furthermore, since the closo-borane is an inorganic conductor, it removes the risk of the battery catching fire while recharging. In other words, it is a material with numerous promising properties.
“The difficulty was establishing close contact between the battery’s three layers: the anode, consisting of solid metallic sodium; the cathode, a mixed sodium chromium oxide; and the electrolyte, the closo-borane,” said co-author Léo Duchêne, a researcher at Empa’s Materials for Energy Conversion Laboratory and a PhD student at UNIGE’s Faculty of Science.
The researchers dissolved part of the battery electrolyte in a solvent before adding the sodium chromium oxide powder. Once the solvent had evaporated, they stacked the cathode powder composite with the electrolyte and anode, compressing the various layers to form the battery.
When the time came to test the battery, the electrochemical stability of the electrolyte was found to withstand three volts — unlike other solid electrolytes previously studied, which have been damaged at the same voltage. The scientists also tested the battery over 250 charge and discharge cycles, after which 85% of the energy capacity was still functional.
The researchers noted that their experiments are still ongoing, as their battery will need to be tested over 1200 cycles before it can be put on the market. In addition, they still have to test the battery at room temperature so they can confirm whether or not dendrites form, while increasing the voltage even more.
Molecular Velcro coating boosts solar cell efficiency
Researchers have developed a new coating layer that enhances the durability and efficiency of...
Predictive AI model enhances solid-state battery design
ECU researchers are working on ways to make solid-state batteries more reliable with the help of...
Boosting performance of aqueous zinc–iodine batteries
Engineers from the University of Adelaide have enhanced aqueous zinc–iodine batteries using...




